QuickMIC® Ultra-rapid antibiotic susceptibility testing
Meet the QuickMIC® system
QuickMIC® is designed to strengthen your lab by providing reliable and precise results at outstanding speed.
- Precise MIC values in 2-4 hours
- Direct from positive blood cultures
- Antibiotic panels for gram-negative bacteria
Loaded with smart features to make lab life better

Improve clinical decisions
Our unique technology utilises a continuous linear antibiotic gradient, which allows for higher resolution, precision and accuracy than methods based on discrete concentrations.
- Increased confidence in MICs from single testing
- High sensitivity around clinical breakpoints (SIR)
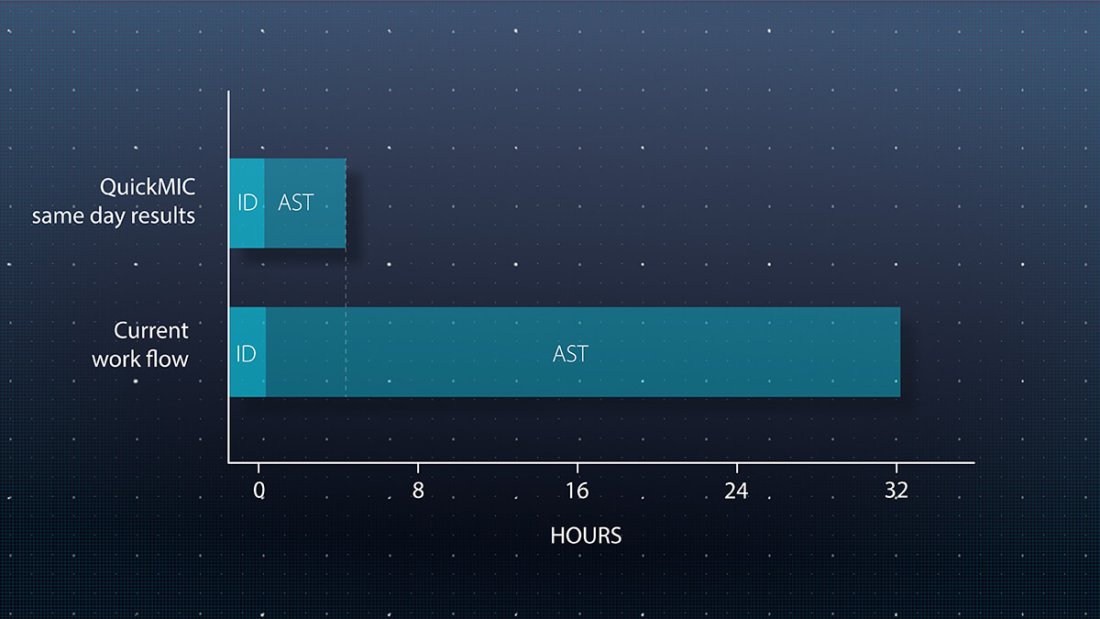
Speed up lab workflows
QuickMIC performs antimicrobial susceptibility testing (AST) directly from positive blood cultures and delivers results within 2-4 hours. Run QuickMIC alongside your preferred rapid ID method to report results to the clinical team even faster.
- Same work shift actionable results
- Minimum hands-on time
- No plate culturing necessary
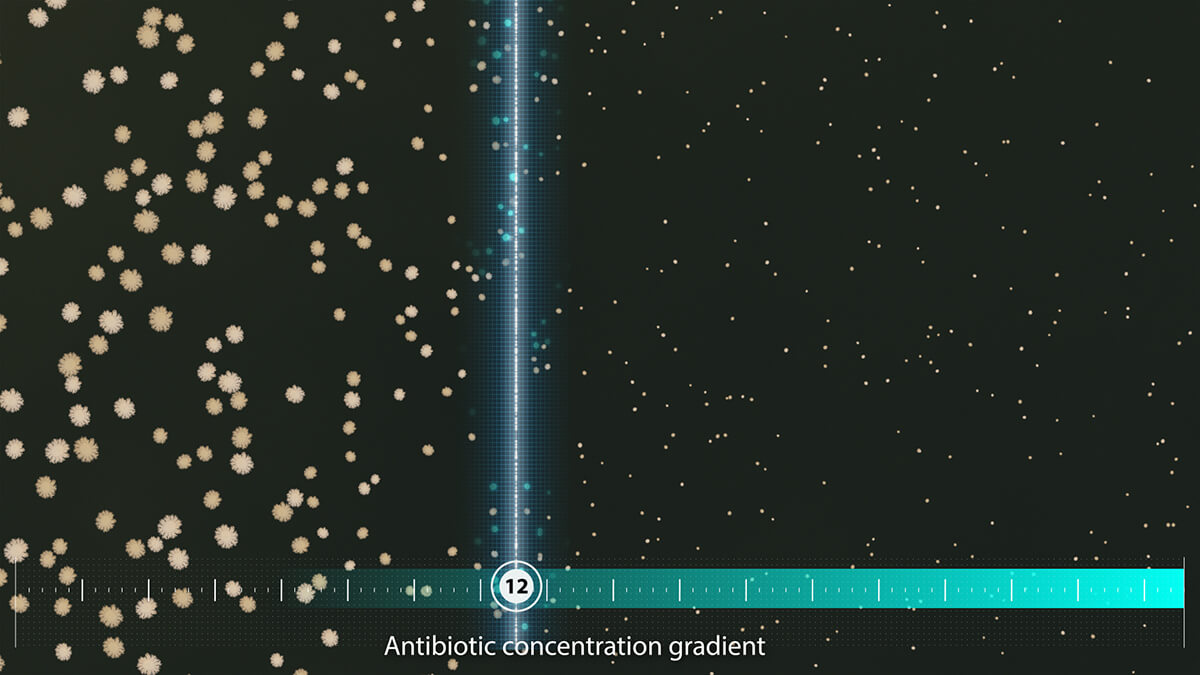
Determine functional resistance in real time
Bacterial microcolonies exposed to a linear antibiotic gradient are followed in real time by live imaging. The software analyses the growth patterns of each microcolony along the linear antibiotic gradient to determine precise MIC values.
- Bacterial growth can be visually monitored throughout the run for each antibiotic
- Phenotypic detection of antibiotic resistance
Learn more about QuickMIC
QuickMIC integrates innovative technology with powerful and intuitive analysis software. Our GN antibiotic panel is tailored for common sepsis-causing bacteria.

